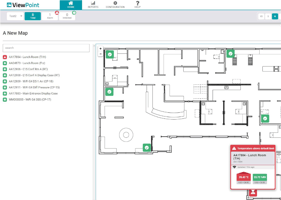
Continuous Monitoring Systems
-
Continuous Monitoring
Precise, Compliant Continuous Monitoring Solutions
Mesa Labs continuous monitoring systems are designed to meet your tracking needs, whether you operate in a highly regulated medical environment or face technically complex manufacturing requirements. We custom engineer our products to provide highly accurate ongoing data collection for key factors like temperature, pressure, and humidity monitoring to ensure that you have the information and insight you need to protect your critical assets and processes.
Distinct Solutions For Ongoing Monitoring Needs
Mesa Labs creates unique solutions that allow continuous monitoring in complex and changing environments. Maintain safety, protect assets, and ensure compliance with decades of expertise by your side.
Customized
Compliant
Regardless of which internal or external standards you follow, find the equipment that meets or exceeds your compliance requirements.
Consultative
We offer both on-site and remote support and consultation. Our consulting services include solution engineering, auditing, and compliance.
Continuous Monitoring Insights
Ensure regulatory compliance and explore the critical importance of continuous monitoring systems.

R&D
Continuous Monitoring Systems: Safeguarding Your Samples in R&D
This expert article will discuss strategies for protecting valuable biological samples throughout the research and development phase.

REGULATORY
Role of Continuous Monitoring Systems in Meeting Regulatory Requirements
Explore the necessity of precise temperature and humidity control in biopharmaceutical environments for regulatory compliance.

QUALITY ASSURANCE
Temperature and Humidity Monitoring: How to Maintain Biopharmaceutical Quality Assurance
Explore the necessity of precise temperature and humidity control in biopharmaceutical environments for regulatory compliance.
Industries
Continuous Monitoring Systems
Our complete line of continuous monitoring sensors and software ensures the flexibility to create a tailored solution that works for your unique application, including meeting or exceeding regulatory and compliance requirements.
Installation & Validation Services
Our global services team helps you meet the ongoing demands of quality control, process validation, regulatory compliance, and citation resolution.
Installation & Qualification
Mesa Labs offers continuous monitoring systems installation, operational and performance qualification, and documentation services to verify that equipment and processes meet industry requirements, user specifications, and compliance needs.
Temperature Mapping
Document how internal and external environmental factors may impact the way products are stored or shipped, with studies designed for any scope from small containers to large warehouses. Study results empower your staff to improve processes and facilities.
Stay Operational & Compliant
We offer remote and on-site calibration and maintenance checks to ensure that your continuous monitoring system continues to function with optimal performance. Our ISO17025-accredited facility offers advanced service and accessory options to help you maintain compliance.